Extractable & Leachable Studies
In the current scenario where E & L studies have become mandatory to meet the safety requirements of medicines and medical devices, Daicel offers the most comprehensive Studies for various portfolio products and a broad range of packaging materials.
Our team also adopts communication and reporting channels through a project management system to match customer specific requirements. We have a team of scientists who are committed to provide the best solutions which meet the needs of pharmaceutical companies.
Daicel offers
- Vast experience of understanding different formulations, plan and design E & L studies in accordance with current regulatory guidelines
- In house state of the art facility for isolation and synthesis of impurities observed above AET in the leachable studies
- Validated methods for both Simulation studies and Leachables studies
- Risk based approach to E & L Study Design keeping the end goal in mind
- Quick TAT’s
- Extractable & Leachables – Quantitative Studies
Why EL testing : Safety and risk to humans
| Risk associated with administration route | Risk of CCS interacting with drug product | ||
|---|---|---|---|
| High | Medium | Low | |
| Highest |
|
|
|
| High |
|
||
| Low |
|
|
|
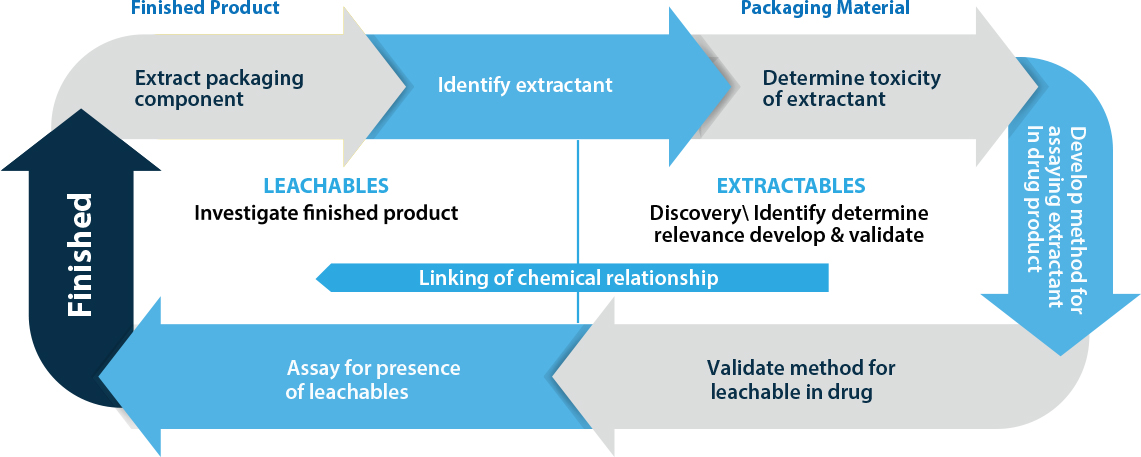
Relationship cycle

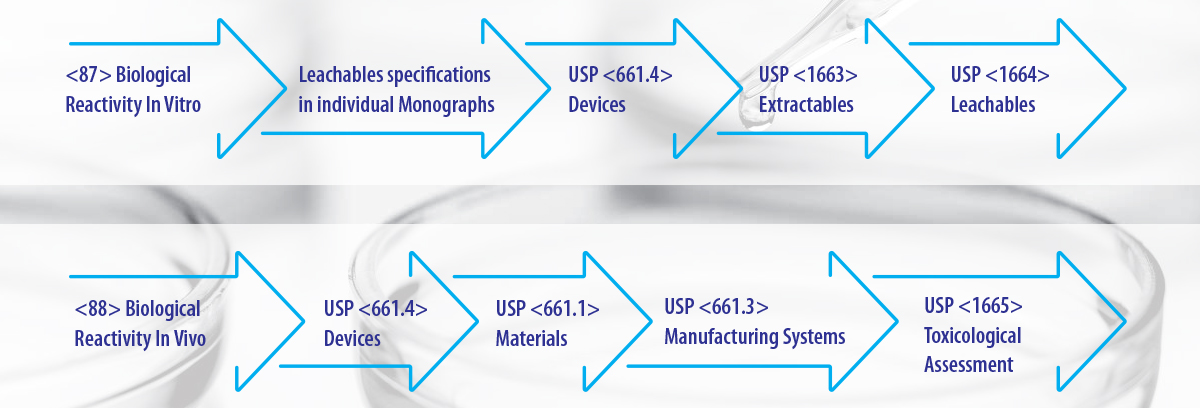
USP Chapters dealing with E & L
Equipped with GC-HS-MS/MS, LC-MS/MS, HRMS, ICP-MS and IC
We ensure compliance with


Key Daicel Capabilities like
Packaging Materials
- Polycarbonate
- Rubber stoppers (Chlorobutyl, Brombutyl)
- Cyclic Olefin copolymer
- Polyvinyl chloride
- Low density polyethylene (LDPE)
- High density polyethylene (HDPE)
- Glass vials
- Closures
- Plungers
Drug Products
- The “4I’s”: Inhalants, Injectables, Infusions, Implantable
- OINDPs (Ophthalmic, pMDI, DPI, Nasal Sprays…)
- Dermal and Topical Applications
- Bio disposables / Single Use Systems
- Vaccines
- Medical Devices
- Label/Ink Migration
- Parenterals
- Peptides/Biologics/Complex Injectables
- Feeding Tubes etc


