Invitro Bioequivalence Studies from Daicel
Invitro Bioequivalence Studies are an increasingly important tool for drug development as they can help drug developers to reduce the cost and time of bringing new drugs to market, while also improving the safety and efficiency of the drug development process.
Daicel can offer Invitro Studies for below mentioned drugs as per FDA PSG recommendation
| Drugs Bind phosphate in GI Tract | Drugs Bind Bile acids in GI Tract | Drugs Bind potassium in GI Tract | Drugs Bind Protein and Bile acids in GI Tract |
|---|---|---|---|
| Calcium acetate | Cholestyramine | Sodium zirconium cyclosilicate | Sucralfate |
| Ferric citrate | Colesevelam HCl | ||
| Ferric oxyhydroxide | Colespol HCl | ||
| Lanthanum carbonate | |||
| Sevelamer carbonate | |||
| Sevelamer HCl |
Invitro BE Studies
- Invitro Equilibrium Binding Assays
- Bile salts Binding Assay
- BSA Binding Assay
- Phosphate Binding Assay
- Potassium Binding Assay
- Kinetic Binding Assays
- Enzymatic (Pepsin) Activity Assay
Daicel established equilibrium curve as expected by FDA
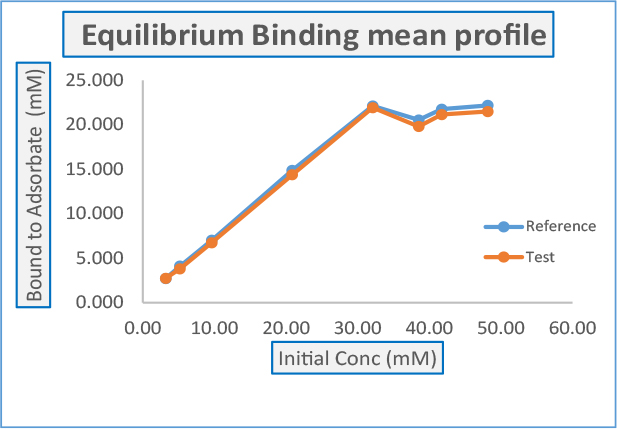
The followings shall be determined for the Bioequivalence studies
- For Invitro Equilibrium Binding Assays
Langmuir binding constant k1 and k2
T/R Ratio should be calculated for k1
Bioequivalence based on (90% CI) for Langmuir binding constant k2 - Kinetic Binding Assays & Enzymatic (Pepsin) Activity Assays
- Equivalence based on: The quantitative comparison between the test and reference formulations


