FDA Special Consideration Studies for
Iron Carbohydrate Complex Drugs
In Vitro characterizations should be conducted on at least three batches of the ANDA and RLD of Iron Carbohydrate Complex Drugs for Special Consideration Studies as mentioned below.
- Catalytic Bleomycin Detectable Iron Assay of Iron Sucrose spiked in Human Serum
- Labile Iron- Kinetic Study of Iron Sucrose in Human Serum
- In Vitro Haemodialysis using In-house developed In Vitro Dialysis System
Our Capabilities and Expertise
- Established methods for Catalytic Bleomycin Detectable Iron (BDI) Assay and Labile Iron-Kinetic Study
- Method Development of In Vitro Equivalence studies
- Method Validations as per ICH Q2 R1
- Statistical Data Analysis for Equivalency Demonstration

In-house developed In Vitro Dialysis System

Multimode Microplate Reader
Target Drugs
- Iron Sucrose
- Ferumoxytol
- Ferric Carboxymaltose
- Other Iron Carbohydrate Drugs
Catalytic Bleomycin Detectable Iron (BDI)
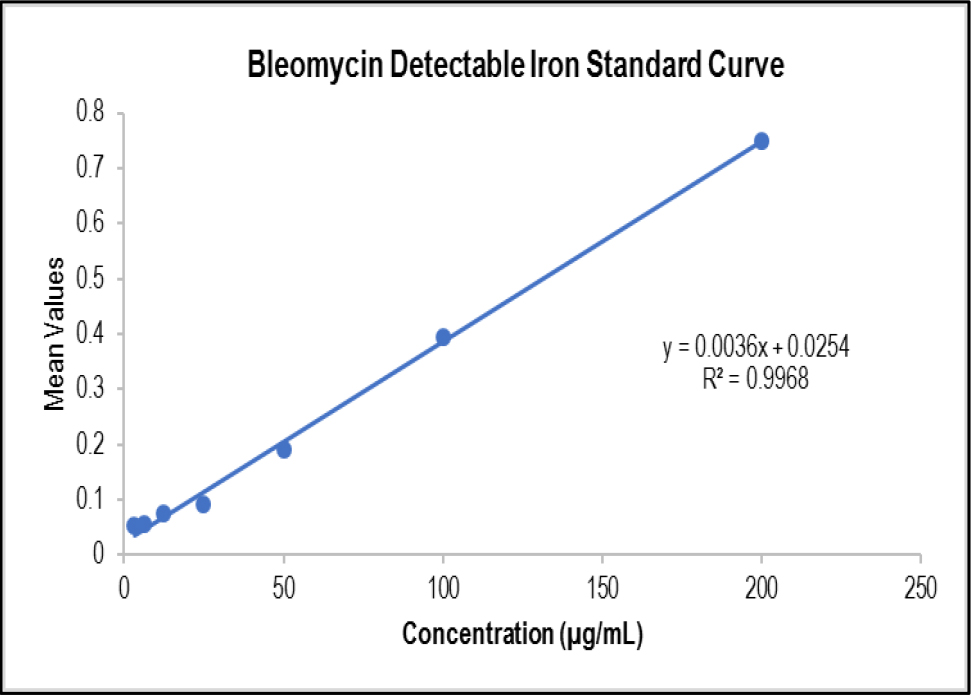
Catalytic Bleomycin Detectable Iron Standard Curve
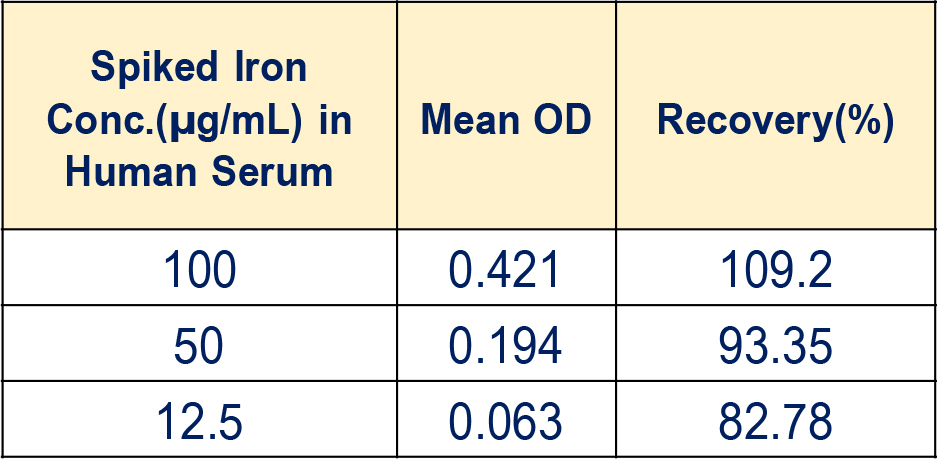
Catalytic Bleomycin Iron Spiked Recovery Concentrations
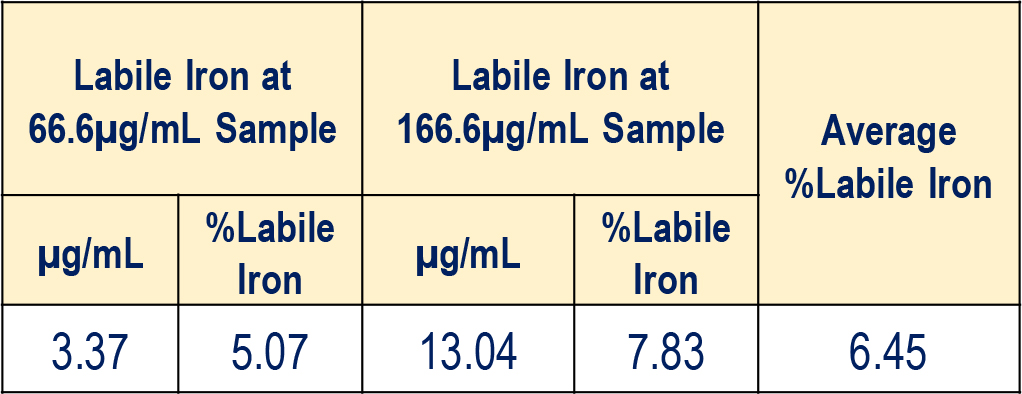
Labile Iron content of Iron Sucrose (kinetic based) spiked in Human Serum
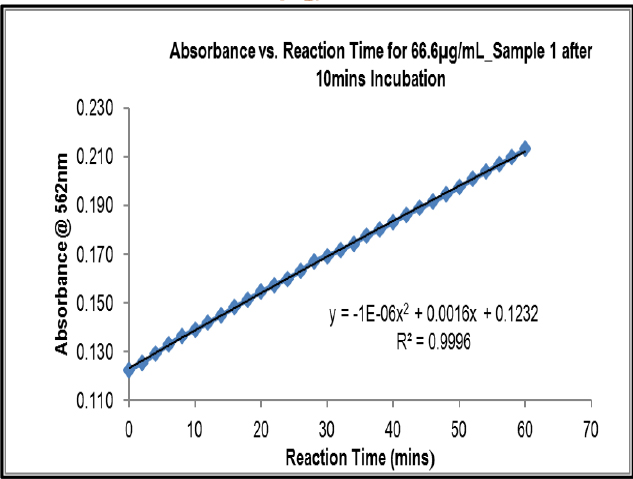
Kinetic Reading after 10 min at 66.6 μg/mL
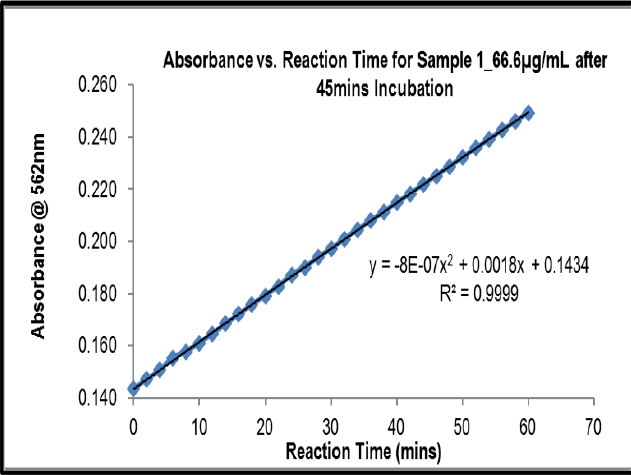
Kinetic Reading after 45 min at 66.6 μg/mL
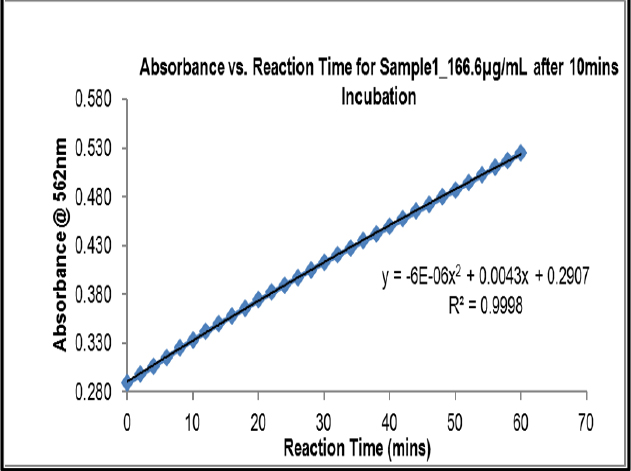
Kinetic Reading after 10 min at 166.6 μg/mL
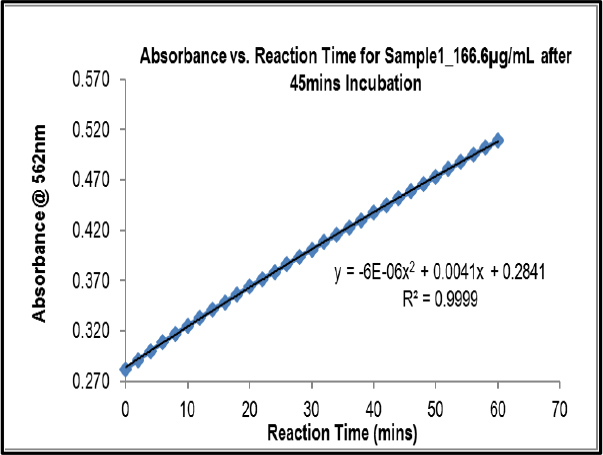
Kinetic Reading after 10 min at 166.6 μg/mL
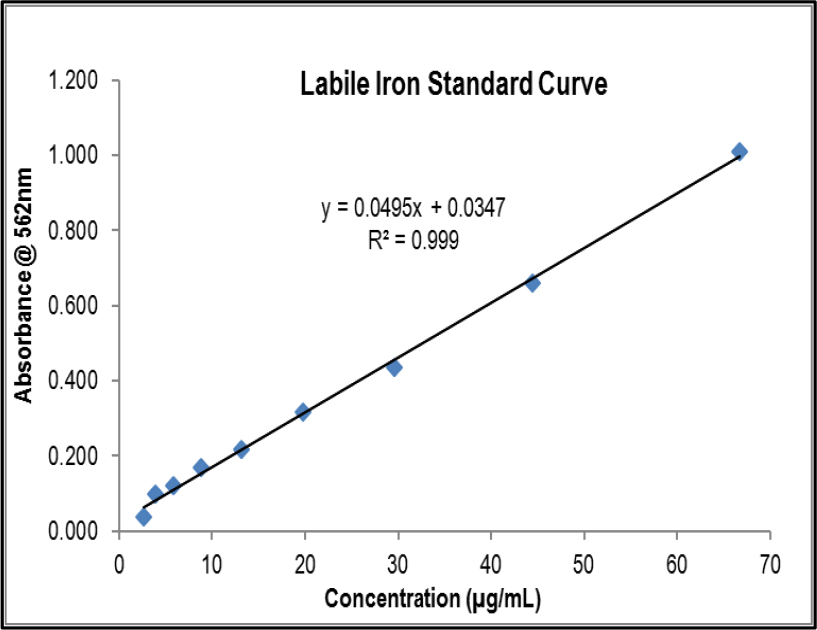
Labile Iron Standard Curve
Labile Iron concentration in the Iron Sucrose Sample at 66.6 and 166.6 μg/mL concentrations